Canon Science Lab
Incandescent and Fluorescent Lighting
We cannot produce sunlight, but we can create illumination that is similar. Examples include incandescent and fluorescent lighting.
Something that emits light is known as a light source.
Light sources can be divided into natural light sources, such as the sun, stars, lightning, and bioluminescence, and artificial light sources, including incandescent lighting, fluorescent lighting, and sodium lamps. They can also be categorized by their light intensity characteristics, i.e., constant light sources that emit the same amount of light over a fixed period of time (for example, the sun and incandescent lighting) and light sources that vary over time. Fluorescent lighting may appear to be constant, but it actually changes in accordance with the frequency of the power source. The human eye is just not capable of detecting such fast variations.
Incandescent Light Shines because of Heat
Incandescent light appears yellowish compared to fluorescent light. This is because incandescent lamps produce light from heat. The filament in an incandescent lamp is what heats up. Filaments are made out of double coils of tungsten, a type of metal. Tungsten has a high electrical resistance, causing it to glow (incandesce) when an electric current flows through. Electric current, through high electrical resistance, results in heat due to the friction between the material and the electrons that are flowing through the material. Tungsten is used for incandescent bulb filaments because it is extremely resistant to melting at high temperatures. It also does not burn, because gas is injected into incandescent bulbs to eliminate all oxygen.
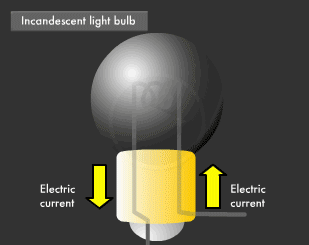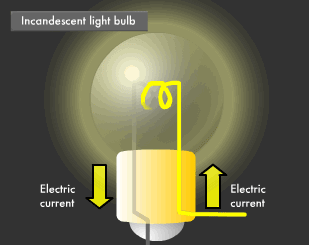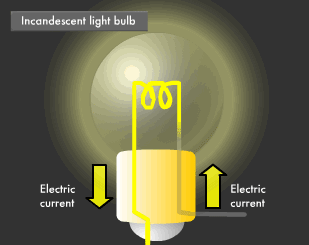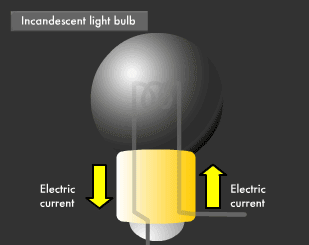
The incandescent lamp was invented by Thomas Edison in 1879. At that time, filaments were carbonized fibers made by smothering a certain type of bamboo grown in Kyoto, Japan, but these days a variety of materials and methods are introduced to produce light bulbs. There are many types of light bulbs, each with their own purpose. For example, there are silica bulbs with silica particles coated electrostatically on their inner surface to vastly improve light transmission and diffusion, krypton bulbs injected with krypton gas (higher atomic weight than the normally used argon gas) to increase brightness, and reflector lamps using highly reflective aluminum on their inner surface.
Fluorescent Light Is more Complicated than It Looks
Fluorescent light, a common form of illumination in offices, has a more complicated light emission mechanism than incandescent light. Ultraviolet rays created within fluorescent lamps are transformed into visible light that we can see. Electrical discharge phenomena and the "excited state" and "ground state" of electrons play an important role here. Let's start with a look at the basic structure of a fluorescent lamp. Fluorescent lamps are slender glass tubes coated with fluorescent material on their inner surfaces.
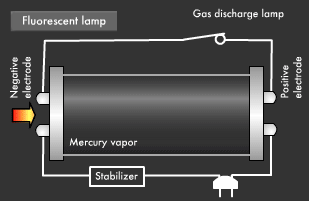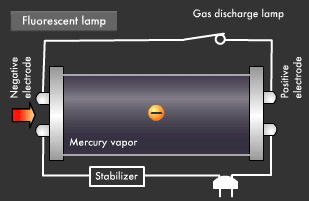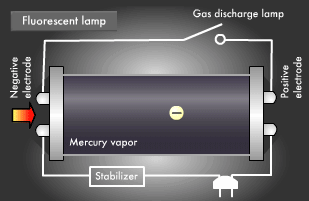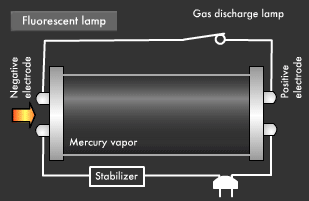
Mercury vapor is injected inside, and electrodes are attached at both ends. When voltage is applied, an electric current flows in the electrodes, causing the filaments on either end to be heated up and start emitting electrons. Next, a small gas discharge lamp inside the fluorescent lamp turns off; electrons are emitted from the electrode and they begin to flow toward the positive electrode. It is these electrons that produce ultraviolet light.
Electrons and Atoms Collide within Fluorescent Lamps
Let's take a closer look at the mechanism by which fluorescent light emits ultraviolet rays. Electrons emitted from the electrode collide with the mercury atoms comprising the vapor inside the glass tube. This causes the mercury atoms to enter an excited state, in which the electrons on the outermost orbit of the atoms and molecules obtain energy, causing them to jump to a higher orbit.
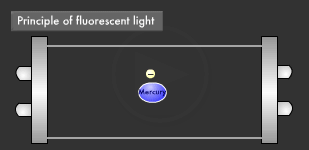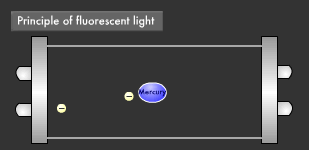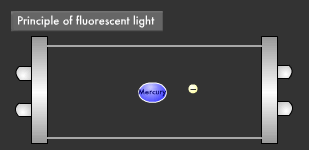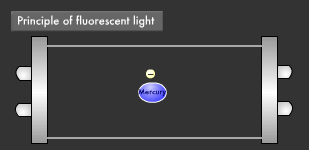
Excited mercury atoms constantly try to return to their former low energy state (ground state), because they are so unstable. When this happens, the energy difference between two orbital levels is released as light in the form of ultraviolet waves. However, since ultraviolet rays are not visible to the human eyes, the inside of the glass tube is coated with a fluorescent material that converts ultraviolet rays to visible light. It is this coating that causes fluorescent lamps to glow white. Fluorescent lamps are not always straight tubes. They come in other forms such as rings and bulbs. Some types of fluorescent lamps have undergone ingenious modifications, such as lamps using a metal line on the outer surface of the tube (rapid start type), eliminating the need for a gas discharge lamp inside.
White LEDs Used in Lighting
The LEDs (light-emitting diodes) used in lighting emit white light similar to that of the sun. White light is created when light's three primary colors — RGB (red, green and blue) — are present. At first, there were only red and green LEDs, but the development of blue LEDs led to the development of white LEDs for use in lighting.
There are two ways to create white LEDs. The first is the "multi-chip method," in which each of the three primary-color LEDs are combined, and the second is the "one-chip method," which combines phosphor and a blue LED. The multi-chip method using three colors requires a balance between brightness and color to realize uniform illumination, and requires that each of the three color chips be equipped with a power circuit.
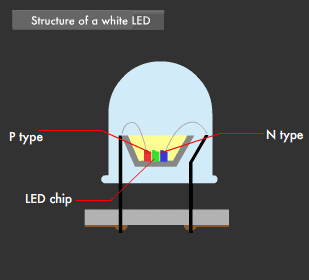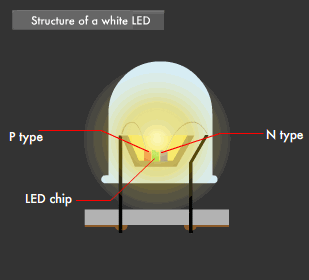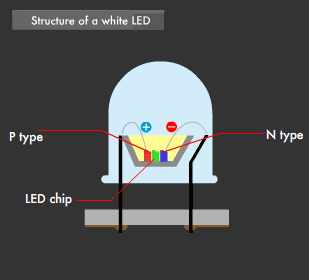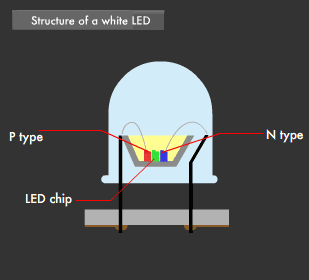
This was the reason behind the development of the one-chip method, which emits a near-white (quasi white) color using a single blue LED and yellow phosphor. This is because blue light and yellow light mixed together appears almost white to the human eye.
Using the one-chip method, white LEDs have been developed that make use of a blue LED in combination with yellow + red phosphor, or green + red phosphor, to achieve a more natural LED-based white light. Furthermore, LEDs that emit near-ultraviolet light (near-ultraviolet light LED: 380-420nm wavelength) have been developed recently and, used as an excitation light source, have led to white LEDs capable of emitting the entire visible light range.
Light Sources Have a "Color Temperature"
In our daily lives, we often notice that the color of clothing as seen under fluorescent lights indoors looks different under sunlight outdoors and that the same food appears more appetizing under incandescent lighting than it does under fluorescent lighting. Have you ever wondered what causes such differences? We see the color of an object when light strikes it and reflects back to our eyes. In short, the colors we perceive change in accordance with the wavelength component of the light source illuminating the objects we see. This results in the above-mentioned differences we perceive in clothing and food illumination.

Differences in color are represented by "color temperature." Color temperature is a numeric value representing chroma rather than the temperature of the light source. All objects emit light when heated to an extremely high temperature. Color temperature indicates what color we would see if we were to heat up an object that reflects no light whatsoever, i.e. a "black body," to a certain temperature. The unit of measurement used in this case is degrees Kelvin. Low-temperature objects appear red, and as they heat up, they start to look blue.
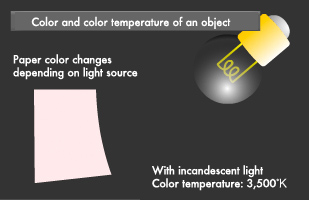
As you can see in the table below, the color temperature of reddish colors is low, while that of bluish colors is high. Color temperature is used for such purposes as setting the color on a computer monitor.
Color Temperature and light sources
| Color temperature | Light source |
|---|---|
| 10,000 | Clear sky |
| 9,000 | Hazy sky |
| 8,000 | |
| 7,000 | Cloudy sky |
| 6,000 | Flash lamp |
| 4,500 | White fluorescent lamp |
| 4,000 | |
| 3,500 | 500-W tungsten lamp |
| 3,000 | Sunrise, sunset |
| 2,500 | 100-W light bulb |
| 2,000 | |
| 1,000 | Candlelight |
