Canon Science Lab
Light in the Natural World
In-addition to man-made light such as incandescent/fluorescent lighting bonfires, and lamps, there are many other forms of light in the natural world. How do you suppose such light phenomena occur?
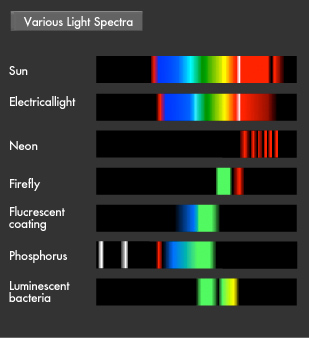
Touching a doorknob may sometimes be accompanied by a zap of static electricity. Occurrences such as this show that the atmosphere is full of electricity. These light- and sound-producing occurrences are called "atmospheric electrical phenomena." Lightning, auroras, and Saint Elmo's Fire are some examples. It is thought that will-o'-the-wisps and other strange events might also be related to atmospheric electrical phenomena. There are even living creatures that emit light, such as fireflies. Light in nature is also a full of mysteries.
What Exactly Are Fluorescence and Phosphorescence?
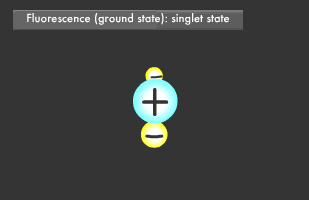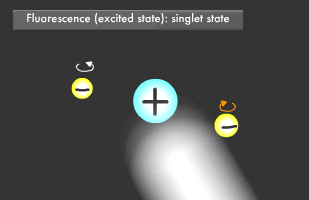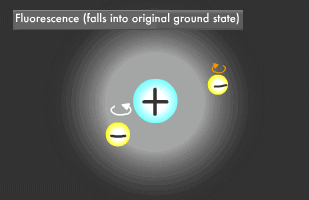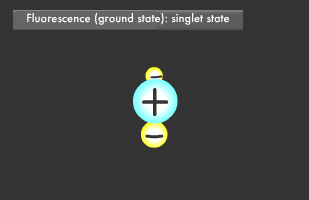
Have you ever been startled by a glowing object in the darkness? Let's start by learning about fluorescence and phosphorescence, either of which was likely the source of the light you saw. Materials that emit light after being illuminated with light or electric beams are called fluorescent or phosphorescent materials. Fluorescent material is used in fluorescent lighting and TV cathode-ray tubes, phosphorescent material in wristwatch faces. These materials absorb light and then emit it at a different wavelength. Fluorescence is what occurs when a material absorbs light and then emits it almost immediately, for about 1/1 billion to 1/100,000 second, while phosphorescent materials glow more slowly, for 1/1,000 to 1/10 second. Fluorescence and phosphorescence are also called "luminescence." Electrons on the outermost orbit of atoms and molecules are what emit light as a general rule. Electrons sometimes obtain energy from an external source, causing them to jump onto a higher orbit. When they later descend to their original energy level, known as their "ground" or "normal" state, the difference of energy between two orbital (energy) levels they fall will be released as light. ("Electromagnetic wave" is generally a more accurate description than "light".) The transition to a higher energy level is called "excitation."
Only two electrons can orbit in a particular energy level. This is called the Pauli Exclusion Principle. Electrons also rotate as they orbit. The two electrons in an energy level will usually rotate in opposite directions (singlet state), though they also might rotate in the same direction (triplet state). In fluorescence, the two excited electrons are in the singlet state, while in phosphorescence, they are in the triplet state. Phosphorescence emits light longer because it takes more time for the electrons to fall to their former singlet ground state.
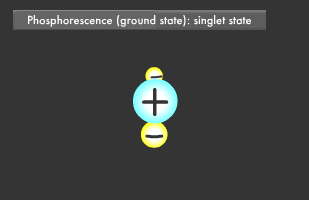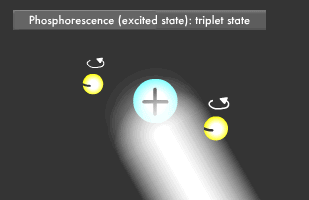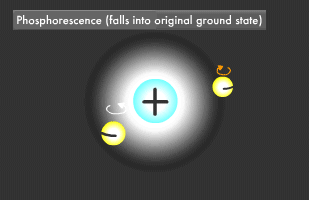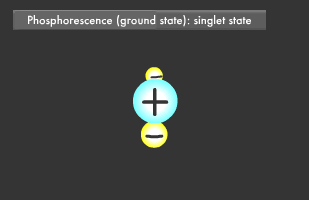
Can You Read a Book by the Light of a Firefly?
Although it resembles fluorescence, the light of a firefly is completely different. Firefly light is caused by the chemical reaction of a material. New molecules in a highly excited state are formed when the material is exposed to oxygen. Light will be emitted when returning to a normal low-energy state or ground state from an excited state. (Since no heat is produced, this is known as "cold light..") The material known as luciferin in the bodies of fireflies oxidizes due to the enzyme luciferase.
This is why fireflies glow when they breathe in and expand their abdomen. The spectrum of the greenish light fireflies produce is not a broad, continuous spectrum as with an electric lamp, but you certainly can make out the words in a book when they approach.
Firefly light plays an important role in unexpected places. One example is genetic recombination. In order to verify whether DNA incorporating a newly inserted gene has properly entered a cell's nucleus, a gene that produces luciferase is also inserted. A dim glow produced by the application of luciferin is proof that the new gene has been properly incorporated.
Do Ships Glow Eerily on Dark Nights?
Seafarers used to witness a mysterious phenomenon back in the days when sailing ships were the only means of sea travel. After encountering tempests and violent thunderstorms, they sometimes saw what appeared to be a pale fire burning at the tip of the mast. Thinking it was a sign of God's blessing, they named the phenomenon Saint Elmo's Fire after the patron saint of sailors. It can also be seen on church steeples, mountaintops, the tips of antenna and other such places. We now know that Saint Elmo's Fire is caused by electric potential differences in the atmosphere.

A spark will jump between plus and minus electrodes spaced at a certain distance when the voltage applied to them is increased. If you look closely, you will notice the section with a high electric field will emit a faint light (corona discharge) before the spark jumps. Saint Elmo's Fire is corona discharge that occurs when thunderclouds cause a sudden increase in electric potential differences in the atmosphere. Corona discharge occurs at an electric potential difference of about 100 V per centimeter.

At about 1,000 V, Saint Elmo's Fire and other phenomena occur, and at 10,000 V or more, spark discharge, including lightning, occurs. Corona discharge occurs particularly easily at the tip of long objects pointed upwards into the atmosphere when a point discharge current is flowing into them.
Is Lightning Static Electricity with an Attitude?
Lightning is an electric discharge caused by electric potential differences in the atmosphere. Thunderclouds (cumulonimbus) are formed by strong updrafts. Water vapor rising into the sky condenses into water droplets and then freezes into ice crystals. Heavy ice crystals that start falling from the top of the thundercloud coalesce with other ice crystals and water droplets, becoming hail as they continue their descent.

At that point, the steadily growing ice crystals and falling hail begin to collide with each other. Some of the water molecules in these ice crystals and hail ionize into H+ and OH- ions. Compared to the large OH- ions, the small H+ ions are more mobile, and those that are higher in temperature are even more mobile. When low-temperature ice crystals and slightly higher-temperature hail rub against each other, the H+ ions transfer to the ice crystals from the highly mobile hail.
As a result, the ascending ice crystals become positively charged and the descending hail negatively charged, causing the buildup of a positive charge at the top and a negative charge in the middle of the thundercloud. In the part of the thundercloud with an air temperature above -10ºC and close to the earth, water droplets adhere to the surface of the hail, forming a water layer. There are now H+ ions within the hail and low mobility OH- ions within its surface water.
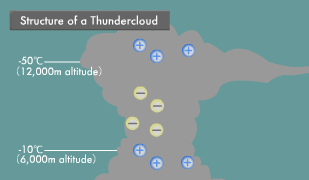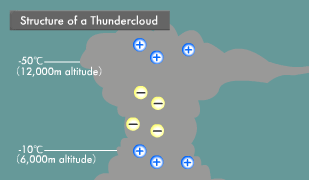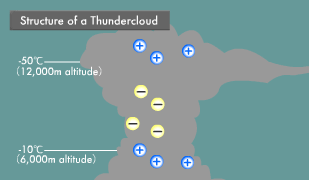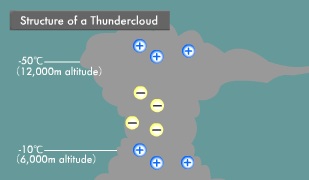
When hail in this state strikes ice crystals, the ice crystals strip off the OH- ion water layer on the surface of the hail and then rise up, leaving the remaining hail with a positive charge. In this manner, the insides of thunderclouds turn into something like an electric power plant, with positive, negative, and positive layers. Within this electric power plant flows a voltage of hundreds of millions of volts and an instantaneous electric current in the tens of thousands of amperes. When this current flows, extremely high temperatures are produced because electric current has an inherent difficulty in passing through the atmosphere. The light generated by the resulting heat appears as lightning.
Are Auroras a Lightshow?
Auroras can be observed near the north and south magnetic poles. They are multicolored curtains of light appearing about 100 km to 1,000 km in the sky. An aurora occurs when charged, high-energy electrons and protons, the main components of the solar wind arriving from the sun, plunge into the atmosphere along magnetic field lines at the North and South poles and collide with nitrogen and oxygen atoms.

The green light (558 nm wavelength) emitted by the excited oxygen atoms can be clearly seen at an altitude of 100 km to 200 km. At an even higher altitude, the 391-nm light emitted by nitrogen atoms can be observed. This light is a kaleidoscope of colors that vary by the hydrogen, oxygen and nitrogen atoms and molecules in the atmosphere. Auroras are observed at the North and South poles because the earth is like one gigantic magnet with the N pole at the South Pole and the S pole at the North Pole attracting charged particles.
