Canon Science Lab
The Earth, Space and Light
Light from space continuously reaches the earth. Let's take a look at the various forms of light arriving from space, including the sunlight that blesses our planet.
Space is filled with waves of various wavelengths. In addition to visible light, there are wavelengths that cannot be seen by the naked eye, such as radio waves and infrared, ultraviolet, X-, and gamma rays. These are collectively known as electromagnetic waves because they pass through space by alternately oscillating between electric and magnetic fields. Light is a type of electromagnetic wave. The electromagnetic waves reaching us consist of only a portion of the visible light, near-infrared rays and radio waves from space because the earth is surrounded by a layer of gases known as the atmosphere. This structure is intimately related to the existence of life on earth.
Does Light Nurture Life?
Life on earth receives the blessings of the light emitted by the sun. The energy that reaches the earth from the sun is about 2 calories per square centimeter per minute, the figure known as solar constant. Calculations based on this figure indicate that every second the sun emits as much energy into space as burning 10 quadrillion (10,000 trillion) tons of coal. The greatest of the sun's gifts to earth is photosynthesis by plants. When a material absorbs the energy of light, the light is changed into heat, thereby raising the object's temperature.
There are also cases where fluorescence or phosphorescence is emitted, but most of the time materials do not change. Sometimes, however, materials do chemically react to light. This is called a "photochemical reaction." Photochemical reactions do not occur with infrared light, but happen primarily when visible light and ultraviolet rays, which have shorter wavelengths, are absorbed.
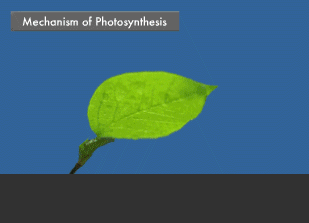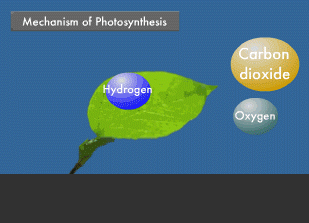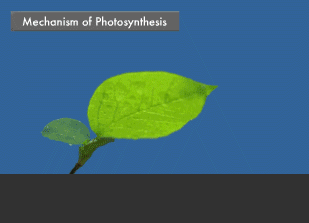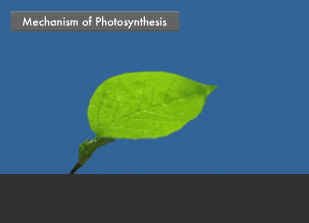
Photosynthesis also is a type of chemical reaction. Plants use the energy of sunlight to synthesize glucose from carbon dioxide and water. They then use this glucose to produce such materials as starch and cellulose. In short, photosynthesis stores the energy of sunlight in the form of glucose. All animals, including humans, live by eating these plants, thereby absorbing the oxygen produced during photosynthesis and indirectly taking in the energy of sunlight. In other words, sunlight is a source of life on earth. We do not yet know the detail of how plants conduct photosynthesis, but in green plants, chloroplasts within cells are known to play a crucial role.
Does the Earth Have Windows?
The earth is surrounded by layers of gases called the atmosphere. Some wavelengths of electromagnetic waves arriving from space are absorbed by the atmosphere and never reach the surface of our planet. Take a look at the following diagram. The earth's atmosphere absorbs the majority of ultraviolet, X-, and gamma rays, which are all shorter wavelengths than visible light.

High energy X- and gamma rays would damage organisms and cells of creatures if they were to reach the earth's surface directly. Fortunately, the atmosphere protects life on earth. Long-wavelength radio waves and infrared rays also do not reach the surface. The electromagnetic waves we can generally observe on the ground consist of visible light, which is difficult for the atmosphere to absorb, near-infrared rays, and some electromagnetic waves. These wavelength ranges are called atmospheric windows. Ground-based astronomical observation employs optical and radio telescopes that take advantage of atmospheric windows. Infrared, X-, and gamma rays, for which atmospheric windows are closed, can only be observed using balloons and astronomical satellites outside the earth's atmosphere.
What Does Invisible Light Do?
Electromagnetic waves are all around us. This includes not only visible light, but also invisible ultraviolet and infrared rays. Infrared rays, with wavelengths longer than visible light, are also a familiar part of electrical appliances. Infrared rays with wavelengths of 2,500 nm and less are called near-infrared rays and are used in TV and VCR remote controllers, as well as in fiber-optic communications. Wavelengths above 2,500 nm are called far-infrared rays and are used in heaters and stoves.
Ultraviolet rays, with wavelengths shorter than visible light, pack high energy that results in sunburns and faded curtains. Ultraviolet rays with wavelengths of 315 nm and less are particularly dangerous because they destroy the DNA within the cells of living creatures. The bactericidal effect of ultraviolet rays is employed for medical implements, but we also know that large doses of these rays can trigger diseases such as skin cancer and have an impact on the entire ecosystem. Ultraviolet rays are suspected in ozone layer destruction, one of the global environmental issues that has been focused on in recent years. Near an altitude of 25 km, there is a rather thick ozone layer of 15 parts per million (ppm).
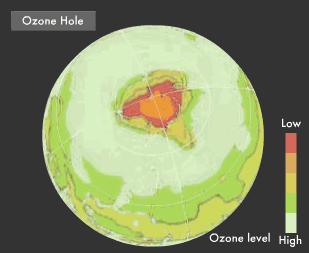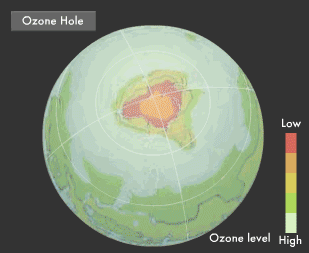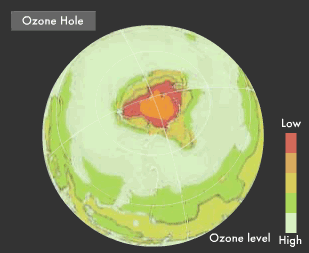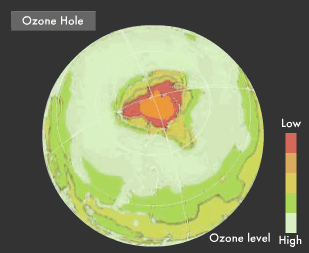
This layer absorbs ultraviolet rays with wavelengths of 350 nm and less, thereby preventing them from reaching the earth's surface, where they would devastate life. Part of the ozone layer is being destroyed by chlorofluorocarbons (CFCs) that are used in refrigerators and air conditioners. "Ozone holes," where the level of ozone is extremely low, can be observed in the Antarctic and other places.
What If Light Does Nasty Things?
"Photochemical smog" is another environmental problem caused by photochemical reactions. Light causes automobile exhaust to chemically react and form irritating materials that produce smog. This occurs around high-traffic roadways on hot, windless days. Automobile engines and other machinery emit nitrogen monoxide (NO). Immediately after entering the atmosphere, NO reacts with oxygen molecules, transforming into nitrogen dioxide, a brownish gas that readily absorbs visible light, causing a photochemical reaction that breaks it down into NO and oxygen atoms. However, this NO once again immediately reacts with oxygen molecules, transforming back into nitrogen dioxide.


Through the process of photolysis, sunlight produces a vast amount of highly reactive oxygen atoms from a small amount of nitrogen dioxide. (A reaction is taking place through which single oxygen molecules consisting of two oxygen atoms are dissociated to form oxygen atoms.) The resulting oxygen atoms bond with oxygen molecules, forming ozone, and with other organic compounds in exhaust gas, forming peroxides. Ozone and peroxides are a source of eye and throat irritation.

Will the Earth Become a Greenhouse?
Another global environmental issue we face is the greenhouse effect. Agriculture and horticulture widely employ greenhouses made of glass or vinyl. These materials allow visible light and near-infrared rays in the wavelength range between 400 nm to 3,000 nm to easily pass through, while making it difficult for infrared rays between 10,000 nm and 20,000 nm to do so. Infrared rays play a central role in the radiation of heat, which is why the inside of a greenhouse heats up. Water vapor such as clouds, carbon dioxide, methane, and Freon are some of the gases that have the same effect on the earth as glass and vinyl do on greenhouses.

They are therefore called greenhouse gases. The specific heats of these gases are larger than that of air, which comprises mostly oxygen and nitrogen, so it becomes difficult to lower the temperature of these gases once it begins to rise. Until today, the earth's temperature has been determined through a precise balance between the atmosphere and the plant and animal ecosystem. However, the greenhouse effect is progressing owing to recent rapid increases in carbon dioxide, and concerns are that this will lead to a rise in atmospheric temperature around the world.
